Folks, BA.5 is coming and it’s going to be bad. Mentally prepare yourself for a big wave this summer.
I don’t know where to put it, but this paper says that compared to peer countries, Canada:
- Did really well on per capita vaccinated, cases, and deaths. (You could argue that we’re fudging the numbers, but our excess deaths rate is also one of the lowest.)
- Had some of the most restrictive mitigation measures.
- Had similar inflation/debt growth, but slightly lower GDP growth.

Treatments
This preprint says that there are a number of mutations which would give SARS-CoV-2 resistance to Paxlovid.
Okay, I need to give some background. Regular antibodies are kind of Y-shaped things which recognize bad actors with their tips. If I understand correctly, nanobodies are sort of like just the tips of the antibodies; they can still gum up the bad guys by glomming on to them. Being smaller, they can get into nooks and crannies that full-on antibodies can’t.
Okay. This paper says that llamas produce really potent nanobodies when vaccinated against COVID-19, and those nanobodies could be potential treatments. Here’s a mass-media article about the study.
Vaccines
A lot happened at the US FDA vaccine subcommittee meeting on 28 June 2002. This liveblog and this Twitter thread cover the meeting; this article gives a summary. (Yes, this is USA and not Canada, but realistically, we’re going to end up with whatever vax the FDA choses.) I’m integrating over multiple sources in this section.
All three vax makers who presented had a monovalent BA.1-based booster and a bivalent with a mix of the BA.1 booster and COVID Classic booster.
- Pfizer
- They said that both their monovalent BA.1 and the bivalent booster worked pretty well against BA.4/BA.5, but that the monovalent worked slightly better. (See this article and this Pfizer press release for more).
- They had data from mouse studies of a BA.4/BA.5-based booster in mice which showed the BA.4/BA.5-based booster (not surprisingly) did better against BA.4/BA.5 than the BA.1-based booster.
- They said they have significant amounts of BA.1 NOW, and are preparing a large amount of BA.4/BA.5. Either could be ready for large-scale rollouts in October (which is what the US FDA is targeting).
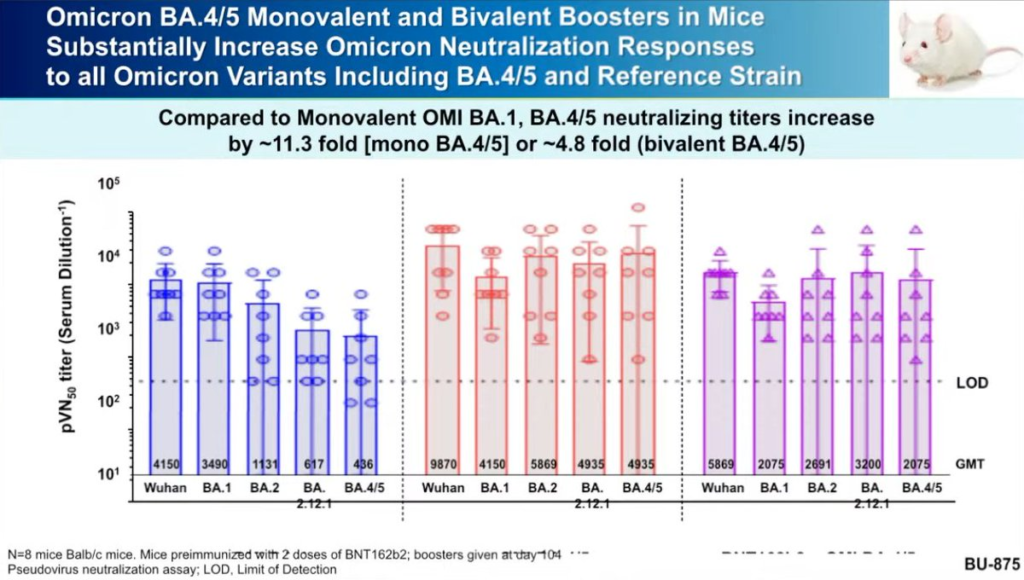
- Moderna
- Moderna had already shown (see this article) that their bivalent vax (mRNA1273.214) is more protective than the monovalent for both Omicron and non-Omicron strains.
- Moderna said could have the BA.1-based vax available in as little as one month. Making BA.4/BA.5-based vax would take about three months.
- Moderna showed data that said that their bivalent COVID Classic/BA.1 vax gave antibodies which lasted longer than their monovalent BA.1-only vax:

- Novavax
- They presented info (this article for more detail) which says that Novavax’ original vax (NVX-CoV2373) generates surprisingly good antibodies against BA.4/BA.5 when used as a booster.
- They also have data on NVX-CoV2515, which is a monovalent BA.1 vax and a bivalent mix of the two. Both give better antibodies against Omicron strains, and they found that the monovalent BA.1 vax was just as good as the bivalent vax. (See this article for more detail.)
- They expect NVX-CoV2515 to be available in 2022Q4. (Note that NVX-CoV2373 is approved in Canada but not the US.)

The subcommittee recommended that vaccine production for the fall be bivalent COVID Classic/Omicron. Interestingly, the committee asked the vax makers to use BA.4/5 and not BA.1. I’m pretty sure that will mean that there won’t be data from humans by then, i.e. they will base their results only on information from human blood in test tubes. (Pfizer did have some mouse data.) I don’t think that’s bad — I think the risk is really low — it just seems like a shift. Maybe they can get a bunch of data in humans fast, we’ll see. (It might also be that we aren’t going to be able to get Phase 3 clinical trials for BA.1 either, so it will all be based on human blood in test tubes…)
Meanwhile, this press release says that WHO is recommending that the fall boosters be BA.1-based.
Buried in this article, Health Canada’s vaccine advisory committee will meet on July 4-5.
This article says that Moderna has applied to Health Canada for approval of its bivalent booster.
This preprint from France found that Sanofi-GSK’s Beta-based booster worked better against COVID Classic, alpha, delta, and BA.1 than an mRNA booster.

This article reports that Pfizer says it will start testing pan-coronavirus vaccines later this year.
This article reports that India has approved Gennova’s self-modifying RNA vaccine for emergency use. This is an interesting story for several reasons:
- It’s the first self-amplifying RNA vaccine to be approved.
- It’s stable at fridge temperatures.
- It’s also a soap opera: Seattle-based company HDT Bio says it licensed Gennova to manufacture the vax and have exclusive rights in India, but that Gennova stole it and claims it’s their own.
The Phase 3 results aren’t out yet, so no word on how effective it is. (You looked shocked. Don’t be. My (probably up-to-date) spreadsheet says that there are fourteen vaxes which are in use which have not published Phase 3 results yet.)
This article says that South Korea has approved a vax jointly developed by the University of Washington and SK bioscience Co Ltd (who will manufacture it). There was a really interesting article on this vax that is unfortunately paywalled. It uses nanoproteins resembling the SARS-CoV-2 spke which are held together in a spikey ball (which is a form that several vax candidates (like Novavax) use, except their proteins are more like on ropes than on sticks. The tethers’ flexible ropey nature lets the human immune system “look” at the proteins from more angles. In the lab, at least, it worked really really well.
This article says that a big vaccine manufacturing centre has opened in Saskatoon.
Transmission
This paper found that infectiousness of aerosols dropped quickly over time, more quickly in low humidity settings than in high-humidity settings.

If I understand the charts from this study right, 25% of people with Omicron had culturable virus nine days after their first positive PCR test. (Presumably, the delay between symptoms and test is more than zero.) My interpretation is that the official guidance that you can stop isolating after five days is complete and utter bullshit.

Pathology
This paper from the US (from March) says that people over 50 who have had COVID-19 infection are 15% more likely to get shingles. (So don’t just get a COVID-19 vaccination, get a shingles vaccination too! And vaccinate your kids against chickenpox!)
From this tweet, which was live-tweeting the US CDC part of the US FDA vax subcommittee meeting, MISC-C appears to happen much less frequently with Omicron than with previous strains.

This paper looked at BMI and COVID risk and found that the relative risk varied a lot with how many shots you’d gotten. (That seems really strange to me.) Being overweight was comparatively bad if you only had one or two shots, but being underweight was comparatively bad if you had more than one shot.

Children
This article from the UK says that little kids’ development was harmed by lockdowns. I would contend that it is disingenuous to say that these reductions are an effect of lockdowns: they are effects of the pandemic. There are many things that happened besides government-mandated movement restrictions – deaths, voluntary movement reductions, job losses, parental Long COVID, etc.

Variants
This report from the UK says that BA.5 is about a 25% growth advantage over BA.2.12.1:

Mitigation Measures
This article reports that Canada is extending its border measures until 30 September 2022.
Long COVID
This article reports on this study which says that women are 22% more likely overall to get Long COVID than men. Interestingly, it’s not the same across all symptoms: men are more likely to have endocrine disorders (like diabetes) and renal disorders.
Recommended Reading
This blog post talks about how BA.5 is coming and will be bad. This article talks about why BA.5 is coming.
This blog post looks at the question of whether female-led countries did better in the pandemic than male-led country. Spoiler: it’s complicated.
This article looks at long-term changes to disease spread as a result of pandemic-induced behaviour changes.
