Pathology
This paper from the USA says that the more physically active people were before catching COVID-19, the milder their case was. (If that sounds familiar, that’s because this paper from September from the USA said the same thing.)
This paper from the USA found that five kids with MIS-C all had variants in a closely related cluster of genes (OAS1, OAS2, or RNASEL). These produce excessive cytokines when stimulated by SARS-CoV-2, and that’s what turns into MIS-C.
Vaccines
This report from the USA says that a bivalent booster cuts your risk of an emergency room visit by about half compared to 2+ primary doses. This report from the USA says that a bivalent booster cuts hospitalization by 73% versus a monovalent primary series. (This blog post talks more about these two studies, if you are interested.)
This paper from the UK found that people who had been hospitalized for COVID-19 had minimal levels of nasal IgA nine months later, and that intramuscular mRNA vaccination didn’t budge those levels at all. This is probably why people get reinfected (and a reason to really try to get nasal vaccines working).
This paper from South Africa found that as the number of exposures (infections or vaccinations) increased, even though antibody levels went up, T-cell levels (CD4 or CD8) didn’t change much.

There have been a number of papers which have shown that myocarditis from vaccination is WAY less dangerous than myocarditis from COVID-19. This paper reports that myocarditis from vaccination is also WAY less dangerous (92% lower mortality risk) than viral mycarditis from viruses other than COVID-19.
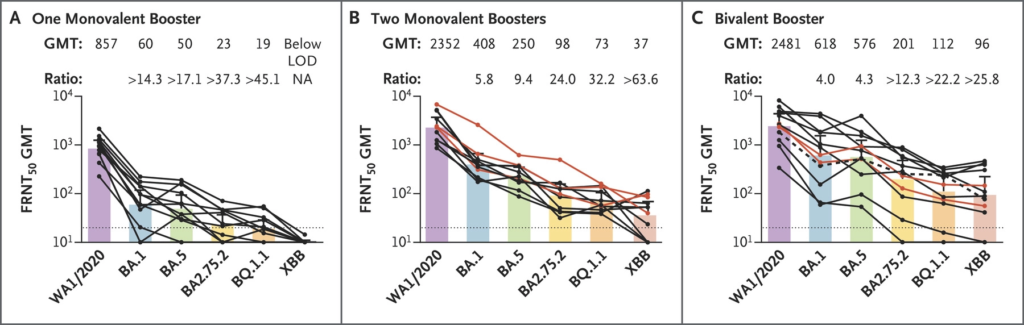
This correspondence from the USA says that blood from people who got one bivalent booster neutralized the scary new “alphabet soup” Omicron variants more than people who got one or two shots of monovalent boosters (especially against the scary R346T mutation variants BA.2.75.2, BQ.1.1, and XBB).
This article says that Canada’s Vaccine Injury Support Program has paid out $2.7M for 50 claims of serious and permanent injuries connected to vaccines.
I’m a big fan of vaccines, I know that there are rare but bad side effects, and I’m all in favour of compensating people for serious and permanent injuries. But only an average of $54,000? That hardly seems like enough.
Transmission
This article from the US talks about COVID-19 in animals. They’ve found COVID-19 in a bunch of common North American mammals. Catching the virus from a skunk isn’t that much of a danger — very few people spend a lot of time really close to skunks — but there is a risk of the virus hopping to an animal, mutating to suit the animal, and then jumping back to humans in a more transmissible form. This might have already happened — there’s hints that Omicron came from spillback from a mouse.
This preprint from Japan says that transmissible COVID-19 can live in human corpses. This preprint from Japan says that dead COVID-19 infected hamsters can transmit COVID-19 to other hamsters, but not if their orifices are plugged.
This release from the UK government announces that they have approved Sanofi/GSK’s vaccine, VidPrevtyn Beta. The most interesting thing about the Sanofi/GSK vax is that it uses the spike from the Beta variant. (I have vague memories that one of the mRNA vendors said (maybe at the FDA presentation in summer 2022?) that their tests with bivalent Classic plus Beta had shown really broad protection, but that it wasn’t as good against the Omicrons as the bivalent which used BA.1, so I am not completely surprised. I’m too lazy today to hunt down the source though.)
According to this Government of Canada page, Canada has a contract with Sanofi to buy up to 76M doses. (Of course, Canada also has a contract to buy up to 76M doses of Medicago, and I haven’t seen any discussion of those doses arriving in the country.)
This press release says that a more people who got this Sanofi/GSK booster after 2 mRNAs had neutralizing levels of antibody in their blood than people who got 3 mRNAs.
You might hear people refer to this paper where the Society for Healthcare Epidemiology of America argues that asymptomatic people should not be tested. However, that’s only half of their recommendation. The other half is “when other infection prevention strategies are in place” (e.g. wearing masks and improved ventilation). In other words, they thought that money/time were better spent on getting other mitigation measures up to snuff.
This paper from Switzerland says that viruses in aerosols are inactivated by acid in the air. They say that influenza A in normal air gets inactivated within minutes, but COVID-19 takes days, but that adding small amounts of acid to the air will kill it much faster.
Long COVID
This case study report says that eight of twelve people with brain fog improved significant with a combo of guanfacine (an ADHD med) and N-acetylcysteine (aka NAC, an antioxidant). Both drugs are already approved, cheap, common, and pretty safe. Yay!!
This study from the USA says that Paxlovid cut Long COVID rates in half.
This study from the USA says that people who were hospitalized and had a positive COVID-19 were at significantly higher risk (10-50% higher) than hospitalized patients who did not have COVID-19 for diabetes, venous disorders, respiratory diseases, and/or fatigue.
Testing
This US CDC advisory now says that, because rapid antigen tests have so many false negatives with Omicron, it requires testmakers tell people to test twice over three days if they have symptoms, and three times over five days if they have no symptoms. This makes the rapid tests not very rapid. 🙁
Variants
This paper from BioNTech researchers in multiple countries says that while some of the alphabet soup variants can evade B-cells easily, it looks like the T cells still target them well.
Treatments
This paper from Australia found that IL-6 receptor antagonists and antiplatelet agents improved COVID-19 survivability, but theraputic anticoagulants, convolescent plasma, and lopinavir-ritonavir did not help. hydroxycholoroquine made survivability worse.
This paper from the UK found that molnupiravir didn’t prevent hospitalizations or death.
Recommended Reading
This article, published in four journals simultaneously, calls for a rethink of random clinical trials, making them less heavyweight. During the pandemic, the UK introduced the RECOVERY framework — which included the IT tools to support it — which told doctors which of the trialed (known) drugs to prescribe, then aggregated information from doctors doing routine clinical care to be able to see which drugs worked better. The RECOVERY framework was hugely successful. It was the study which proved that dexamethasone (which was already generic, so cheap!) was very useful against COVID-19.
One of the reasons this worked was, ironically, because they had no clue what would work. Doctors were making scientifically-informed guesses, using drugs which had already been approved for other purposes. RECOVERY took the most promising of those and randomized them up.
One big advantage of RECOVERY is that it used pre-existing drugs. No drug company would have ever paid for a random clinical trial of dexamethasone because the patent protections had already expired. And, once dexamethasone was shown to be useful against COVID-19, everyone in the world could immediately start using it — because it had already been approved.
RECOVERY-style analysis of “routine care” is a hugely, hugely important area of medicine right now. Even if you don’t read the article, you should be aware of it.
This blog posting isn’t about COVID-19, but it’s important: it talks about using fast DNA testing as a diagnostic tool.
This article talks about mucosal vaccines.
