Variants
The current data, while confusing and somewhat contradictory, at least shows that our wave is not getting lots worse, and might even be going down. This seems really strange, when compared to how many other jurisdictions have gotten hammered.
According to this blog post, the US had large shares of BA.2.12.1 — which shares a key mutation (at L452) with BA.4/5 — and so the population might have better immunity to BA.4/5.
Eyeballing the US CDC variant tracking page, it looks like BA.2.12.1 had about a 60% share.
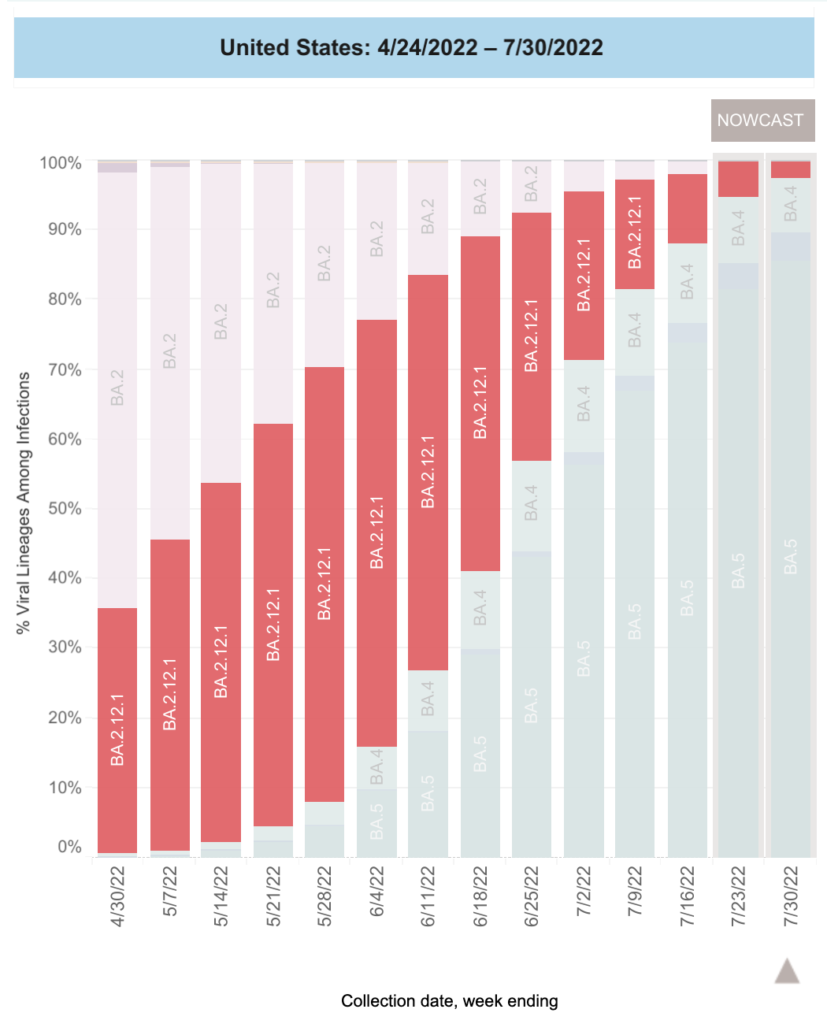
BA.2.12.1 didn’t have as big a share of infections as the US did, but it was still sizeable. Eyeballing the Government of Canada’s COVID-19 epidemiology update page, it looks like it had about a 50% share:
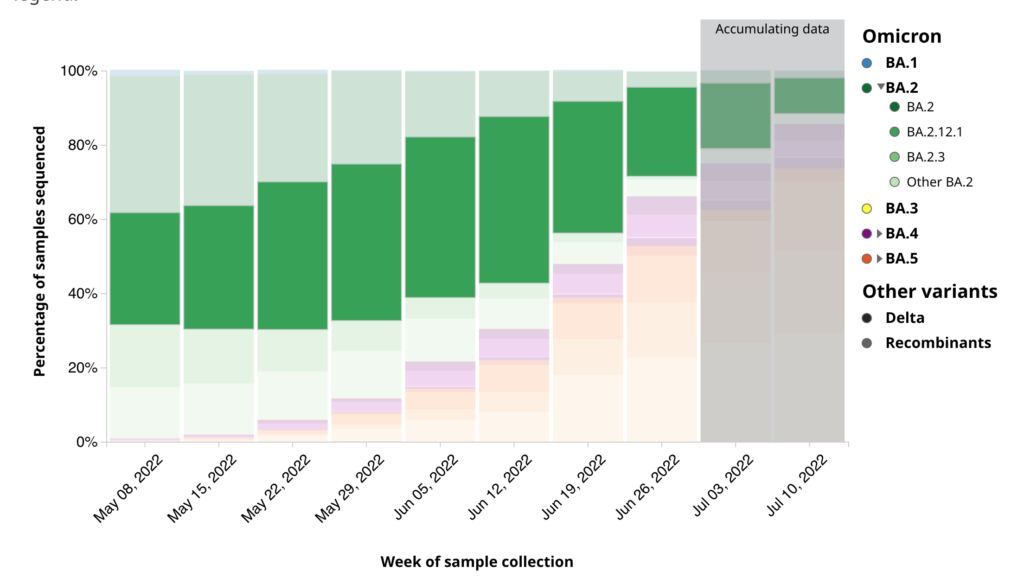
Even now, there’s a lot of BA.2.12.1 in Canada compared to other places:

I am starting to get hopefully optimistic about September. In most of the past waves, I was able to see the next wave coming on the horizon. Right now, maybe BA.5 has peaked (maybe?), but I don’t see a scary variant on the horizon. That doesn’t mean we will not have another variant hit us when BA.5 peters out (which I think will be in early or mid-September) — Omicron only gave us a month of warning — but we might get lucky in September.
Yes, BA.2.75 is out there, but studies like in this preprint from the USA make me think it’s not that scary. (The preprint says that BA.2.75 is more resistant to bebtelovimab, which is bad, but that it is less resistant to immunity from vaccination.)
Treatments
This article from the USA on Paxlovid/Molnupiravir rebound says that the percentages of people who had been given one of the drugs and then later were infected, infected with symptoms, or hospitalized were:
| Drug | Effect | 7-day | 30-day |
| Paxlovid | infection | 3.53% | 5.40% |
| Paxlovid | symptomatic infection | 2.31% | 5.87% |
| Paxlovid | hospitalizaton | 0.44% | 0.77% |
| Molnupiravir | infection | 5.86% | 8.59% |
| Molnupiravir | symptomatic infection | 3.75% | 8.21% |
| Molnupiravir | hospitalizaton | 0.84% | 1.39% |
However, once they controlled for the populations, they said there were no significant differences between Paxlovid and Molnupiravir. They also said that patients with COVID-19 rebound had significantly higher prevalence of underlying medical conditions than those without.
This preprint from the USA, on the other hand, found that 12% of the patients treated with Paxlovid had a rebound.
Long COVID
This article talks about a Long COVID treatment that failed its Phase 2 test, but the company thinks the drug still shows promise: the standard deviation was just so high that their study was too small to give significance on the primary measure the study was designed for (a blood measure of fatigue called phosphocreatine recovery rate (PCr)). They did find significant improvement on self-reported mental and physical fatigue, and think that justifies a Phase 3 study.
Interestingly, the the researchers had expected the test subjects to have a badness measure of about N for PCr, but it was actually way worse, almost 2N.
This report from the USA says that the risk to children under 18 who have had COVID-19 is higher than those who have not as follows:
- ~double the risk of acute pulmonary embolism;
- ~double the risk of myocarditis and cardiomyopathy;
- 87% higher risk of venous thromboembolic event;
- 32% higher risk of acute and unspecified renal failure;
- 23% higher risk of type 1 diabetes.
These might be under-estimates.
This paper says that most people get their smell back within eight weeks:

This article reports on as-yet unpublished study which found that people over 60 who still had loss of smell after three months were 1.5x as likely to have cognitive impairments as those who never lost it or recovered it. This was true regardless of how sick the patients got.
This article says that the rate of Long COVID might be overestimated, in part because it’s beastly hard to study. Different studies have different definitions, different timescales, different variants during the study period, and different populations. Many studies do not have control populations. Many studies are based on surveys with self-reporting. (I saw one study that appalled me: it basically asked people, “have you been tired at any time in the past year?”, “have you had a headache any time in the past year?” and did not have a control group that it asked these questions of.) Many (most?) studies don’t look at severity. (There’s a big difference between everything tasting like there was a bit too much pepper in everything and having everything taste like burned rotting tar-covered liver. (Yes, I hate liver.))
Pathology
This paper from Japan found a marker (myosin light chain 9) which predicts how bad the disease is going to be in that patient. This is actually very encouraging! Not only could this marker be used to guide treatment, but it also might give clues for better treatments.
This paper from Australia found that the virus doesn’t infect the epithelial cells in children’s noses as well as adults’ noses, but that Omicron infected those cells better than previous variants.
Vaccines
This report from the USA says that side effects from a second booster are milder on average than from a first booster.
Buried in this article from the USA is word that Pfizer and Moderna say that they will be ready to ship bivalent COVID Classic/BA.5 vax to the USA in early September. (I have not heard about which vax formulation Canada is going to get, or when, but it’s likely that we our doses/deliveries will be very close to what the southern behemoth will get.)
This preprint from the USA says that the Vaxart oral vaccine (in a very small study) gave higher IgA (mucosal) antibodies than infection does.
This paper from Ontario found that vaccination rates among First Nations, Inuit and Métis were lower than for the general population, but that testing rates were higher. Infection rates were similar to the overall provincial and local rates.
Mitigation Measures
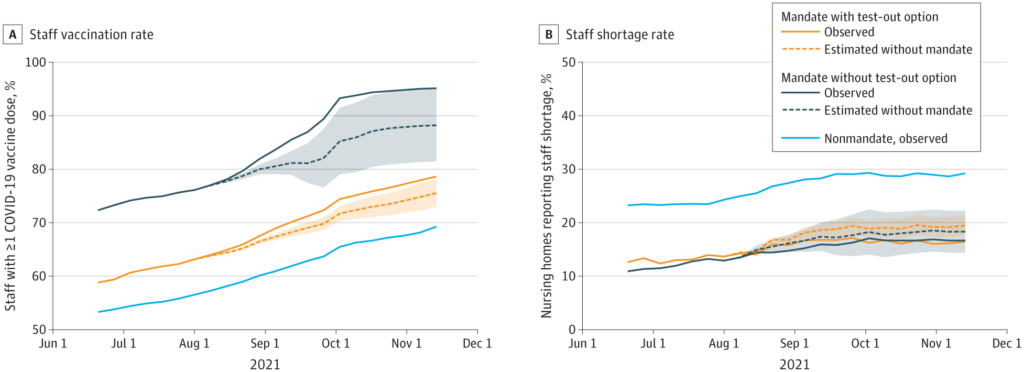
This paper from the USA found that states which mandated vaccine coverage for nursing home workers had higher vaccination rates and lower rates of staff shortages than states without mandates.
Treatments
Something that I had not realized is that, as this article says, bebtelovimab (the only monoclonal antibody which still works against BA.5) only had ONE customer — the US Government. It is not available in Canada. So apparently we have zero monoclonal antibody treatments here. And the Health Canada page on treatments doesn’t even list Molniravir. I guess it’s Paxlovid or bust. 😬
Unexpected Consequences
This article says that online crime went up significantly during the pandemic, including a 21% increase in cybercrime-related harassing and threatening behaviour violations between 2019 and 2021.
Recommended Reading
This blog post gives an update on mucosal vaccines.
