How to get Best Results
There are some things you can do to make your vaccination work better:
- This paper from USA (2022-02-09) reports that 90 minutes of light/moderate exercise immediately after getting an immunization can boost antibody levels without causing more/worse side effects.
- This paper from UK (2021-12-04) says you should get vaccinated in the morning.
- This paper from USA (2024-01-16) says that you should switch which arm you get vaccinated in. Unfortunately, this paper from Germany (2023-08-11) says that same-arm vaccination is better!
Comparing Vaccines
So.. just how good are the different vaccines? Note that you can’t reeeeally do a straight comparison of one vax to another because the trials measured different things with different populations and different variant mixes at different times. As a good article in The Economist says, “the gap in reported efficacy may say more about the trials than about the vaccines themselves.”
Not only that, but some trials had such low numbers of severe cases even in the placebo branch that it was really hard to draw good conclusions.
With that disclaimer, I present data below. Other sources include:
- This (long) blog post/article has an AMAZING list of vax results through 26 January 2022.
The US CDC has a chart that is similar to the one I maintain below.
I have split off effectiveness to COVID Classic and to pre-Omicron variants (Alpha, Beta, Gamma, Delta) because the page was getting too long. There is also a page on how well non-coronavirus vaccines are against COVID. Surprisingly, many give some protection.
Efficacy against Omicron
| Brand | Location | Against | When | # doses | VE |
| Pfizer or AZ | England | hospitalization | omicron wave | 2 or 3 | 81% |
| Pfizer or AZ | England | hospitalization | omicron wave | 3 | 88% |
| J&J | South Africa | hospitalization after 0-13 days | 2021-Nov-15 to 2021-Dec-20 | 2 | 63% |
| J&J | South Africa | hospitalization after 14-27 days | 2021-Nov-15 to 2021-Dec-20 | 2 | 84% |
| J&J | South Africa | hospitalization after 1-2 months | 2021-Nov-15 to 2021-Dec-20 | 2 | 85% |
| Moderna | California | infection at 14-90 days | 2021-12-06 to 2021-12-23 | 2 | 30.4% |
| Moderna | California | infection | 2021-12-06 to 2021-12-23 | 3 | 62.5% |
| Pfizer | California | Emergency Department admission, <3 months post vax | Dec 01, 2021 through Jan 11, 2022 | 2 | 60% |
| Pfizer | California | Emergency Department admission, over 3 months post vax | Dec 01, 2021 through Jan 11, 2022 | 2 | 41% |
| Pfizer | California | Emergency Department admission, <3 months post vax | Dec 01, 2021 through Jan 11, 2022 | 3 | 78% |
| Pfizer | California | Emergency Department admission, over 3 months post vax | Dec 01, 2021 through Jan 11, 2022 | 3 | 48% |
| Pfizer | California | hospital admission | Dec 01, 2021 through Jan 11, 2022 | 2 | 68% |
| Pfizer | California | hospital admission | Dec 01, 2021 through Jan 11, 2022 | 2 | 89% |
| UK vaxes combined | UK | mortality, 25 weeks later | early 2022 | 2 | 59% |
| UK vaxes combined | UK | mortality | early 2022 | 3 | 95% |
| Moderna | USA | infection | 6 Dec 2021 to 31 Dec 2021 | 2 | 44.0% |
| Moderna | USA | infection at 14-60 days | 6 Dec 2021 to 31 Dec 2021 | 3 | 76.1% |
| Moderna | USA | infection at >60 days | 6 Dec 2021 to 31 Dec 2021 | 3 | 47.4% |
| Moderna | USA | hospitalization | 6 Dec 2021 to 31 Dec 2021 | 3 | >99% |
| mRNA | USA | hospitalization | 11 Mar 2021 to 14 Jan 2022 | 2 | 65% |
| mRNA | USA | hospitalization | 11 Mar 2021 to 14 Jan 2022 | 3 | 86% |
| Pfizer | Finland | hospitalization 14-90 days later | 1 Jan 2022 to 19 Feb 2022 | 2 | 91% |
| Pfizer | Finland | hospitalization 91-180 days later | 1 Jan 2022 to 19 Feb 2022 | 2 | 76% |
| Pfizer | Finland | hospitalization 14-60 days later | 1 Jan 2022 to 19 Feb 2022 | 3 | 95% |
| mostly mRNA | USA | emergency visits after 2 months | 26 Aug 2021 to 22 Jan 2022 | 3 | 87% |
| mostly mRNA | USA | hospitalization after 2 months | 26 Aug 2021 to 22 Jan 2022 | 3 | 91% |
| mostly mRNA | USA | emergency visits after 4 months | 26 Aug 2021 to 22 Jan 2022 | 3 | 66% |
| mostly mRNA | USA | hospitalization after 4 months | 26 Aug 2021 to 22 Jan 2022 | 3 | 78% |
| Pfizer | Hong Kong | severe disease or death | 31 Dec 2021 to 8 Mar 2022 | 2 | 88.2% |
| Coronavac | Hong Kong | severe disease or death | 31 Dec 2021 to 8 Mar 2022 | 2 | 74.1% |
| Pfizer or Coronavac | Hong Kong | severe disease or death | 31 Dec 2021 to 8 Mar 2022 | 3 | 98.1% |
| Prior infection only | Qatar | symptomatic BA.2 | 23 Dec 2021 to 21 Feb 2022 | N/A | 46.1% |
| Pfizer (Moderna “comparable”) | Qatar | symptomatic BA.2 after “several” months | 23 Dec 2021 to 21 Feb 2022 | 2 | ~0% |
| Pfizer (Moderna “comparable”) | Qatar | symptomatic BA.2 | 23 Dec 2021 to 21 Feb 2022 | 3 | 52.2% |
| Pfizer (Moderna “comparable”) | Qatar | symptomatic BA.2 | 23 Dec 2021 to 21 Feb 2022 | 2 plus prior infection | 55.1% |
| Pfizer (Moderna “comparable”) | Qatar | symptomatic BA.2 | 23 Dec 2021 to 21 Feb 2022 | 3 plus prior infection | 77.3% |
| Pfizer (Moderna “comparable”) | Qatar | severe, critical, or fatal BA.2 | 23 Dec 2021 to 21 Feb 2022 | any combo of vax and/or infection | >70% |
| J&J | USA | ER/Urgent care visits | Dec 2021 to Mar 2022 | 1 | 24% |
| J&J | USA | ER/Urgent care visits | Dec 2021 to Mar 2022 | 2 | 54% |
| J&J/mRNA | USA | ER/Urgent care visits | Dec 2021 to Mar 2022 | 1 J&J and 1 mRNA | 79% |
| mRNA | USA | ER/Urgent care visits | Dec 2021 to Mar 2022 | 3 | 83% |
| J&J | USA | hospitalization | Dec 2021 to Mar 2022 | 1 | 31% |
| J&J | USA | hospitalization | Dec 2021 to Mar 2022 | 2 | 67% |
| J&J/mRNA | USA | hospitalization | Dec 2021 to Mar 2022 | 1 J&J and 1 mRNA | 78% |
| mRNA | USA | hospitalization | Dec 2021 to Mar 2022 | 3 | 90% |
| Pfizer | USA | hospitalization of 12-18 y/os | 1 Jul 2021 to 17 Feb 2022 | 2 | 40% |
| Pfizer | USA | “critical COVID” of 12-18 y/os | 1 Jul 2021 to 17 Feb 2022 | 2 | 79% |
| Pfizer | USA | “non-critical COVID” of 12-18 y/os | 1 Jul 2021 to 17 Feb 2022 | 2 | 20% |
| Pediatric Pfizer | USA | hospitalization of 5-11 y/os | 1 Jul 2021 to 17 Feb 2022 | 2 | 68% |
| Pfizer | Denmark | infection after 14 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 37.0% |
| Pfizer | Denmark | infection after 121 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 9.8% |
| Moderna | Denmark | infection after 14 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 37.9% |
| Moderna | Denmark | infection after 121 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 13.2% |
| Pfizer | Denmark | hospitalization after 14 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 50.5% |
| Pfizer | Denmark | hospitalization after 121 days | 28 Dec 2021 to 15 Feb 2022 | 2 | 51.6 |
| Pfizer | Denmark | infection after 14 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 47.9% |
| Pfizer | Denmark | infection after 121 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 40.5% |
| Moderna | Denmark | infection after 14 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 47.7% |
| Moderna | Denmark | infection after 121 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 37.9% |
| Pfizer | Denmark | hospitalization after 14 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 88.8% |
| Pfizer | Denmark | hospitalization after 121 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 66.2% |
| Moderna | Denmark | hospitalization after 14 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 90.2% |
| Moderna | Denmark | hospitalization after 121 days | 28 Dec 2021 to 15 Feb 2022 | 3 | 77.3% |
| mRNA | Germany | symptomatic infection in adults | 25 Dec 2021 to 7 Feb 2022 | 2 | 70% |
| mRNA | Germany | symptomatic infection in 12-17 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 88.3% |
| mRNA | Germany | symptomatic infection in 18-59 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 64.7% |
| mRNA | Germany | symptomatic infection in ≥60 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 81.6% |
| mRNA | Germany | hospitalization | 25 Dec 2021 to 7 Feb 2022 | 3 | 94.4% |
| mRNA | Germany | hospitalization in 12-17 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 90·5% |
| mRNA | Germany | hospitalization in 18-59 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 89.9% |
| mRNA | Germany | hospitalization in ≥60 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 95.9% |
| mRNA | Germany | severe illness | 25 Dec 2021 to 7 Feb 2022 | 3 | 97.5% |
| mRNA | Germany | severe illness in 18-59 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 96·2% |
| mRNA | Germany | severe illness in ≥60 y/os | 25 Dec 2021 to 7 Feb 2022 | 3 | 97·7% |
| mRNA | USA PROTECT | infection, 5-11 y/o | Jul 2021 to Feb 2022 | 2 | 31% |
| mRNA | USA PROTECT | infection, 12-18 y/o, 14-149 days | Jul 2021 to Feb 2022 | 2 | 59% |
| mRNA | USA PROTECT | infection, 12-18 y/o, ≥150 days days | Jul 2021 to Feb 2022 | 2 | 62% |
| Pfizer | USA | infection, 5-11 y/o | 1 Jul 2021 to 17 Feb 2022 | 2 | 68% |
| Pfizer | USA | infection, 12-18 y/o, 2-22 weeks | 1 Jul 2021 to 17 Feb 2022 | 2 | 42% |
| Pfizer | USA | infection, 12-18 y/o, 23-44 weeks | 1 Jul 2021 to 17 Feb 2022 | 2 | 36% |
| Pfizer | USA | hospitalization, 12-18 y/o | 1 Jul 2021 to 17 Feb 2022 | 2 | 20% |
| Pfizer | USA | life support or death, 12-18 y/o | 1 Jul 2021 to 17 Feb 2022 | 2 | 79% |
| Pfizer | Israel | infection | 3 Jan 2022 to 18 Feb 2022 | 4 | 45% |
| Pfizer | Israel | symptomatic infection (relative to 3 doses) | 3 Jan 2022 to 18 Feb 2022 | 4 | 55% |
| Pfizer | Israel | hospitalization (relative to 3 doses) | 3 Jan 2022 to 18 Feb 2022 | 4 | 68% |
| Pfizer | Israel | severe disease (relative to 3 doses) | 3 Jan 2022 to 18 Feb 2022 | 4 | 62% |
| Pfizer | Israel | death (relative to 3 doses) | 3 Jan 2022 to 18 Feb 2022 | 4 | 74% |
| Pfizer | Finland | hospitalization after 14-90 days | 1 Jan 2022 to 19 Feb 2022 | 2 | 91% |
| Pfizer | Finland | hospitalization after 14-60 days | 1 Jan 2022 to 19 Feb 2022 | 3 | 95% |
| Pfizer | Finland | hospitalization after 91-180 days | 1 Jan 2022 to 19 Feb 2022 | 2 | 76% |
| mRNA | Denmark | infection after 14-30 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 39.9% |
| mRNA | Denmark | infection after >120 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 4.7% |
| mRNA | Denmark | infection after 14-30 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 57.6% |
| mRNA | Denmark | infection after >120 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 52.8% |
| mRNA | Denmark | infection after 14-30 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 39.8% |
| mRNA | Denmark | infection after >120 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 13.2% |
| mRNA | Denmark | infection after 14-30 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 55.2% |
| mRNA | Denmark | infection after >120 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 49.9% |
| mRNA | Denmark | hospitalization after 14-30 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 94.4% |
| mRNA | Denmark | hospitalization >120 days, over 60 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 77.3% |
| mRNA | Denmark | hospitalization after 14-30 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 62.4% |
| mRNA | Denmark | hospitalization after >120 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 2 | 65.9% |
| mRNA | Denmark | hospitalization after 14-30 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 89.3% |
| mRNA | Denmark | hospitalization after >120 days, 12-59 y/o | 21 Dec 2021 to 31 Jan 2022 | 3 | 33.3% |
| Pfizer | California | hospital admission after >9 months | 1 Dec 2021 to 6 Feb 2022 | 2 | 41% |
| Pfizer | California | ER admission after >9 months | 1 Dec 2021 to 6 Feb 2022 | 2 | 31% |
| Pfizer | California | hospital admission after <3 months | 1 Dec 2021 to 6 Feb 2022 | 3 | 85% |
| Pfizer | California | hospital admission after >3 months | 1 Dec 2021 to 6 Feb 2022 | 3 | 55% |
| Pfizer | California | ER admission after <3 months | 1 Dec 2021 to 6 Feb 2022 | 3 | 77% |
| Pfizer | California | ER admission after >3 months | 1 Dec 2021 to 6 Feb 2022 | 3 | 53% |
| mRNA | California prisons | infection | 24 Dec 2021 to 14 Apr 2022 | 2 | 14.9% |
| mRNA | California prisons | infection | 24 Dec 2021 to 14 Apr 2022 | 3 | 43.2% |
| mRNA | California prisons | breakthrough infection, after pre-Delta infection | 24 Dec 2021 to 14 Apr 2022 | 2 | 47.8% |
| mRNA | California prisons | breakthrough infection, after pre-Delta infection | 24 Dec 2021 to 14 Apr 2022 | 3 | 61.3% |
| mRNA | California prisons | breakthrough infection, after Delta infection | 24 Dec 2021 to 14 Apr 2022 | 2 | 73.1% |
| mRNA | California prisons | breakthrough infection, after Delta infection | 24 Dec 2021 to 14 Apr 2022 | 3 | 86.8% |
| Pfizer | Israel | infection, kids 5-11 | 23 Nov 2021 to 7 Jan 2022 | 1 | 17% |
| Pfizer | Israel | infection, kids 5-11 | 23 Nov 2021 to 7 Jan 2022 | 2 | 51% |
| Pfizer | Israel | symptomatic infection, kids 5-11 | 23 Nov 2021 to 7 Jan 2022 | 1 | 18% |
| Pfizer | Israel | symptomatic infection, kids 5-11 | 23 Nov 2021 to 7 Jan 2022 | 2 | 48% |
| Arcturus | Vietnam | severe cases | “Delta and Omicron” | 2 | 95.3% |
| Arcturus | Vietnam | infection | “Delta and Omicron” | 2 | 55.0% |
| Pfizer | Southern California | BA.1 ER admission | 27 Dec 2021 to 4 Jun 2022 | 2 | 29% |
| Pfizer | Southern California | BA.1 hospitalization | 27 Dec 2021 to 4 Jun 2022 | 2 | 40% |
| Pfizer | Southern California | BA.1 ER admission, <3 months after vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 74% |
| Pfizer | Southern California | BA.1 ER admission, >3 months after vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 65% |
| Pfizer | Southern California | BA.1 hospitalization <3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 80% |
| Pfizer | Southern California | BA.1 hospitalization >3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 76% |
| Pfizer | Southern California | BA.2 ER admission | 27 Dec 2021 to 4 Jun 2022 | 2 | 16% |
| Pfizer | Southern California | BA.2 hospitalization | 27 Dec 2021 to 4 Jun 2022 | 2 | 56% |
| Pfizer | Southern California | BA.2 ER admission <3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 59% |
| Pfizer | Southern California | BA.2 ER admission >3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 5% |
| Pfizer | Southern California | BA.2 hospitalization <3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 74% |
| Pfizer | Southern California | BA.2 hospitalization >3 months post-vax | 27 Dec 2021 to 4 Jun 2022 | 3 | 70% |
| whatever, mostly mRNA | USA | (BA.1) hospitalization <120 days post-vax | “BA.1 period” | 3 | 92% |
| whatever, mostly mRNA | USA | (BA.1) hospitalization >=120 days post-vax | “BA.1 period” | 3 | 85% |
| whatever, mostly mRNA | USA | (BA.2) hospitalization <120 days post-vax | BA.2 period | 3 | 69% |
| whatever, mostly mRNA | USA | (BA.2) hospitalization >=120 days post-vax | BA.2 period | 3 | 52% |
| whatever, mostly mRNA | USA | (BA.2/BA.2.12.1) hospitalization >=120 days post vax for people over 50 | BA.2/BA.2.12.1 period | 3 | 55% |
| whatever, mostly mRNA | USA | (BA.2/BA.2.12.1) hospitalization >=7 days post vax for people over 50 | BA.2/BA.2.12.1 period | 4 | 80% |
| whatever, mostly mRNA | USA | (BA.1) hospitalization >=150 days post-vax | BA.1 period | 2 | 61% |
| whatever, mostly mRNA | USA | (BA.2/BA.2.12.1) hospitalization >=150 days post-vax | BA.2/BA.2.12.1 period | 2 | 24% |
| whatever, mostly mRNA | USA | (BA.2/BA.2.12.1) ER visit >=120 days post-vax | BA.2/BA.2.12.1 period | 3 | 32% |
| whatever, mostly mRNA | USA | (BA.2/BA.2.12.1) ER visit >7 days post-vax | BA.2/BA.2.12.1 period | 4 | 66% |
| Pfizer | Singapore | PCR or rapid test confirmed infection, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 1 | 13.6% |
| Pfizer | Singapore | PCR confirmed infection, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 1 | 24.3% |
| Pfizer | Singapore | hospitalization, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 1 | 42.3% |
| Pfizer | Singapore | PCR or rapid test confirmed infection, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 2 | 36.8% |
| Pfizer | Singapore | PCR confirmed infection, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 2 | 65.3% |
| Pfizer | Singapore | infection, 5-11 y/os | 21 Jan 2022, through 8 Apr 2022 | 2 | 82.7% |
| Pfizer | Hong Kong | mild or moderate disease in 20-59 y/os | 31 Dec through 16 Mar 2022 | 2 | 35·1% |
| CoronaVac | Hong Kong | mild or moderate disease in 20-59 y/os | 31 Dec through 16 Mar 2022 | 2 | 25·1% |
| Pfizer | Hong Kong | mild or moderate disease in 20-59 y/os | 31 Dec through 16 Mar 2022 | 3 | 73·5% |
| CoronaVac | Hong Kong | mild or moderate disease in 20-59 y/os | 31 Dec through 16 Mar 2022 | 3 | 51·0% |
| Pfizer | Hong Kong | severe disease in over 20-59 y/os | 31 Dec through 16 Mar 2022 | 2 | 96·3% |
| CoronaVac | Hong Kong | severe disease in over 20-59 y/os | 31 Dec through 16 Mar 2022 | 2 | 91·7% |
| Pfizer | Hong Kong | severe disease in 60-69 y/os | 31 Dec through 16 Mar 2022 | 2 | 91.1% |
| CoronaVac | Hong Kong | severe disease in 60-69 y/os | 31 Dec through 16 Mar 2022 | 2 | 79·3% |
| Pfizer | Hong Kong | severe disease in over 80 y/os | 31 Dec through 16 Mar 2022 | 2 | 86·9% |
| CoronaVac | Hong Kong | severe disease in over 80 y/os | 31 Dec through 16 Mar 2022 | 2 | 58·2% |
| Pfizer | Hong Kong | death in over 80 y/os | 31 Dec through 16 Mar 2022 | 2 | 90·3% |
| CoronaVac | Hong Kong | death in over 80 y/os | 31 Dec through 16 Mar 2022 | 2 | 63·0% |
| Pfizer | Hong Kong | severe/fatal disease, all ages | 31 Dec through 16 Mar 2022 | 3 | 98·6% – 99·0% |
| CoronaVac | Hong Kong | severe/fatal disease, all ages | 31 Dec through 16 Mar 2022 | 3 | 95·4% – 98·8% |
| various | Portugal | hospitalization, BA.2 | 25 Apr to 10 Jun 2022 | 3 | 93% |
| various | Portugal | hospitalization, BA.5 | 25 Apr to 10 Jun 2022 | 3 | 77% |
| various | Portugal | death, BA.2 | 25 Apr to 10 Jun 2022 | 3 | 94% |
| various | Portugal | death, BA.5 | 25 Apr to 10 Jun 2022 | 3 | 88% |
| Moderna or Pfizer | Singapore | infection NB: fast waning | 27 Dec 2021 to 10 Mar 2022 | 3 | 31.7% to 41.3% |
| Moderna or Pfizer | Singapore | severe disease NB: minimal waning | 27 Dec 2021 to 10 Mar 2022 | 3 | 87.4% |
| Sinovac or Sinopharm | Singapore | severe disease | 27 Dec 2021 to 10 Mar 2022 | 3 | 69.6%. |
| Pfizer | South Africa | hospitalization during BA.1/BA.2 period at 3-4 months | 15 Nov 2021, to 28 Feb 2022 | 2 | 56.3% |
| Pfizer | South Africa | hospitalization BA.4/BA.5 period at 3-4 months | 15 Apr to 24 Jun 2022 | 2 | 47.4% |
| Pfizer | South Africa | hospitalization BA.1/BA.2 period at 3-4 months | 15 Nov 2021, to 28 Feb 2022 | 3 | 50.0% |
| Pfizer | South Africa | hospitalization BA.4/BA.5 time period at 3-4 months | 15 Apr to 24 Jun 2022 | 3 | 46.8% |
| Moderna | USA | infection (BA.1) 14-30 days after boost | 1 Jan 2022 to 30 Jun 2022 | 3 | 97.5% |
| Moderna | USA | infection (BA.2) 14-30 days after boost | 1 Jan 2022 to 30 Jun 2022 | 3 | 82.0% |
| Moderna | USA | infection (BA.24/5) 14-30 days after boost | 1 Jan 2022 to 30 Jun 2022 | 3 | 72.4% |
| Moderna | USA | infection (BA.5) 14-30 days after boost | 1 Jan 2022 to 30 Jun 2022 | 4 | 30.8% |
| Moderna | USA | hopitalization (BA.4/5) 14-30 days after boost | 1 Jan 2022 to 30 Jun 2022 | 4 | 88.5% |
| Moderna | USA | infection, 2-5 y/os | B.1.1.529 era | 2 | 36.8% |
| Moderna | USA | infection, 6-23 month olds | B.1.1.529 era | 2 | 50.6% |
| mRNA (monovalent) | USA | infection, <120 days after boost | BA.1/BA.2 | 3 | 79% |
| mRNA (monovalent) | USA | infection, <120 days after boost | BA.4/BA.5 | 3 | 60% |
| mRNA (monovalent) | USA | infection, >120 days after boost | BA.1/BA.2 | 3 | 41% |
| mRNA (monovalent) | USA | infection, >120 days after boost | BA.4/BA.5 | 3 | 29% |
| almost all mRNA | Ontario | hospitalization or death, over age 50, 7-59 days after dose | 2 Jan 2022 to 1 Oct 2022 | 3 | 91-98% |
| almost all mRNA | Ontario | hospitalization or death, over age 50, >240 days after dose | 2 Jan 2022 to 1 Oct 2022 | 3 | 76-87% |
| almost all mRNA | Ontario | hospitalization or death, over age 50, 7-59 days after dose | 2 Jan 2022 to 1 Oct 2022 | 4 | 92-97% |
| almost all mRNA | Ontario | hospitalization or death, over age 50, >120 days after dose | 2 Jan 2022 to 1 Oct 2022 | 4 | 86-89% |
| 10 µg Pfizer | Qatar | infection in children immediately after 2nd dose | Omicron period | 2 | 49.6% |
| 10 µg Pfizer | Qatar | infection in children after 3 months | Omicron period | 2 | ~0.0% |
| 10 µg Pfizer | Qatar | infection, children 5 to 7 y/o | Omicron period | 2 | 46.3% |
| 10 µg Pfizer | Qatar | infection, children 8 to 11 y/o | Omicron period | 2 | 16.6% |
| 30 µg Pfizer | Qatar | infection, children 12-14 | Omicron period | 2 | 35.6% |
| 30 µg Pfizer | Qatar | infection, children 15-17 | Omicron period | 2 | 20.9% |
| mRNA | Germany | infection, children 5-11 | Omicron period | 2 | 41.9% |
| Germany | symptomatic infection, children 5-11 | Omicron period | 2 | 38·7% | |
| Germany | hospitalization, children 5-11 | Omicron period | 2 | 75·3% | |
| Pfizer | Hong Kong | infection | 1 Mar 2022 to 15 Apr 2022 | 2 | ~0% |
| CoronaVac | Hong Kong | infection | 1 Mar 2022 to 15 Apr 2022 | 2 | ~0% |
| Pfizer | Hong Kong | infection | 1 Mar 2022 to 15 Apr 2022 | 3 | 41·4% |
| Pfizer | Hong Kong | symptomatic infection | 1 Mar 2022 to 15 Apr 2022 | 3 | 50·9% |
| CoronaVac | Hong Kong | infection | 1 Mar 2022 to 15 Apr 2022 | 3 | 32·4% |
| CoronaVac | Hong Kong | symptomatic infection | 1 Mar 2022 to 15 Apr 2022 | 3 | 41·6% |
| 2 CoronaVac+1 Pfizer | Hong Kong | infection | 1 Mar 2022 to 15 Apr 2022 | 3 | 55·8% |
Dose Interval
What is the optimal interval?
It is my understanding that in most vaccines, you want a fairly long interval between dose 1 and dose 2. The immune system needs some time to learn and train from the first dose.
Why then, are were the recommended intervals so short initially? Because the vaccine makers were in a real hurry. They did the absolute shortest interval that they thought they could get away with.
There have been a lot of people who have had longer intervals since then. It was a bit of a calculated gamble to do so before the data was in, but it was apparently a pretty safe bet, since all other vaccines did better with a longer dose interval.
Since then, there have been a few studies.
- This study of Pfizer in people over 80 found that they generated 2.5x as many antibodies with a 12-week interval than with a 4-week interval.
- This study of AZ found that antibodies were higher with a 12-week dose interval than an interval shorter than six weeks.
- This study of AZ found that antibodies were higher the longer the dosing interval was. They studied up to 44 weeks.
- This study of Pfizer found that neutralizing antibodies were higher for a long dosing interval (6-14 weeks) compared to a short interval (3-4 weeks).
- This study of Pfizer found that a 16-week interval was better than a 4 week interval.
- This study of Pfizer and Moderna found that antibody levels were higher in people who had a 6 to 7 week interval than in people who had a 3 to 4 week interval.
There also have been literally millions of people who have had a longer interval. For example, in BC, most of the 2.3M people who have gotten a second dose (as of 18 July 2021) have had a dose interval longer than 7 weeks. (Mine was ten weeks; my partner’s eleven.) The UK has also had a long dose interval.
Waning Effectiveness
There have been a bunch of studies documenting a significant decrease in effectiveness against infection over time. There is a small drop against hospitalization and death, but not nearly as large.
It is not entirely surprising that the vaccines are less effective against infection but still very good against severe disease. Antibodies are the first line of defence, and they are kind of designed to go away when they are not needed, but to get called back in force when there is an infection. It takes a few days for a new antibody army to get raised, however, which gives time for infection to take hold.
The first Pfizer clinical trials said that the vaccine efficacy after only one dose was 92%, but this re-examination of the clinical data gives much better numbers:

I believe I saw another paper which said that the efficacy declined quite a bit after five weeks, but I would have to dig a bit harder to find that the paper.

This preprint did a meta-analysis of a bunch of waning studies. They found that vaccine effectiveness dropped a lot between 1 and 6 months after the second dose:
| against | ages | change |
| asymptomatic infection | all | -18.5% |
| asymptomatic infection | older | -19.9% |
| symptomatic infection | all | -25.4% |
| symptomatic infection | older | -32.0% |
| severe disease | all | -8.0% |
| severe disease | older | -9.7% |


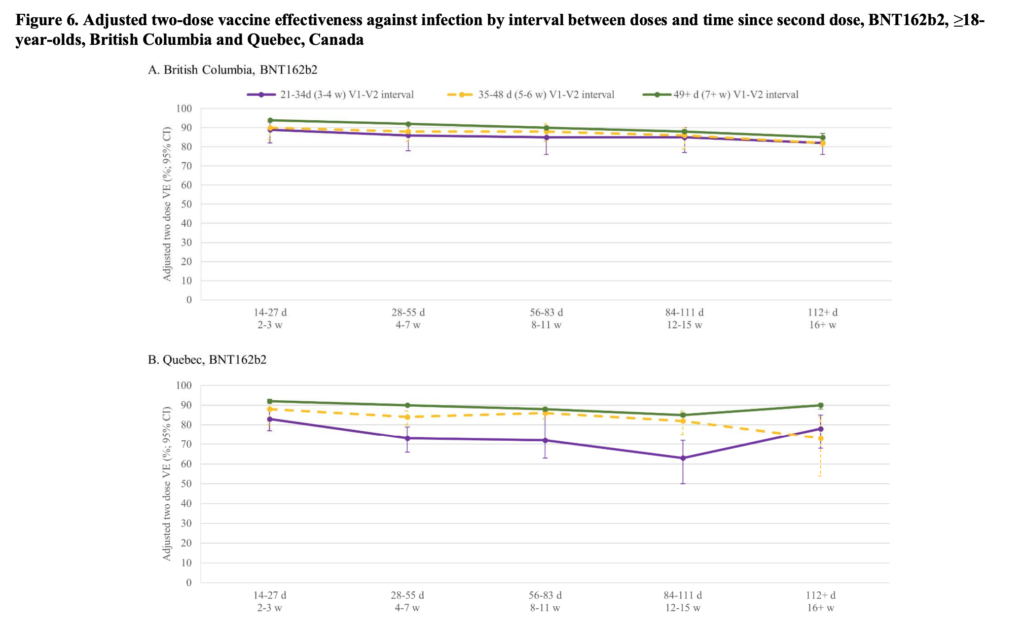
This study from BC and Quebec showed a lot less waning — more like 10% than 20-30% — for long dose intervals than for short dose intervals:
This preprint reports waning of two doses of AZ in Scotland and Brazil. The vaccine effectiveness against severe disease was:
| 2-3 weeks | 14-15 weeks | 18-19 weeks | |
| Scotland | 83·7% | 75·9% | 63·7% |
| Brazil | 86·4% | 59·7% | 42·2% |
Note that there are lot of papers on waning effectiveness, I just got bored writing them all down.
Boosters
This publication from Public Health England says that the vaccine effectiveness was 93.1% for those over 50 with AZ+AZ+Pfizer and 94.0% for Pfizer+Pfizer+Pfizer.
This big study which looked at Pfizer, Moderna, AZ, J&J, Novavax, CureVac, and Valneva as boosters for AZ/AZ or Pfizer/Pfizer found that (broadly speaking), the more mRNA, the better. (See my 2 Dec 2021 analysis for more detail and nuance.)
<there have been a bunch more booster studies, they all show that a booster increases effectiveness against infection, at least in the short term… I need to add them in…>
This preprint says that the J&J boosters (i.e. second dose) in South Africa does well. Its effectiveness against infection was:
- 63% effective 0-13 days after the second dose
- 84% effective 14-27 days days after the second dose
- 85% effective 1-2 months days after the second dose
This preprint says that for Pfizer doses in the USA:
- effectiveness against infection dropped from 85% during the first month after dose2 to 49% >= 7 months after dose2;
- effectiveness against hospitalization after dose2 was 90% and did not wane significantly;
- effectiveness against infection was 88% after dose3;
- effectiveness against hospitalization was 97% after dose3;
- relative effectiveness (vs. unvaccinated) against infection after dose3 was 75%;
- relative effectiveness (vs. unvaccinated) against hospitalization after dose3 was 70%.
